Researchers from Keio University School of Medicine in Tokyo have conducted an early clinical trial demonstrating the potential of the Parkinson’s disease drug ropinirole in treating amyotrophic lateral sclerosis (ALS). ALS, also known as Lou Gehrig’s disease, is a fatal motor neuron disease that leads to a progressive loss of muscle control. The trial, reported in the journal Cell Stem Cell, showed that ropinirole was safe to use in ALS patients and delayed disease progression by an average of 27.9 weeks.
The study involved 20 patients with sporadic ALS, meaning they did not carry genes associated with the disease, and had an average ALS duration of 20 months. The trial was double-blinded for the initial 24 weeks, during which neither the patients nor the doctors knew who was receiving ropinirole and who was receiving a placebo. After this period, all patients who wished to continue were given ropinirole openly for the following 24 weeks. Due to various factors, including the COVID-19 pandemic, the study had a reduced number of participants, with only seven of the ropinirole-treated patients and one of the placebo-followed-by-ropinirole-treated patients monitored for the full year. However, no safety concerns were reported, and all dropouts were unrelated to the drug’s safety.
Throughout the trial, the researchers assessed multiple measures to determine the drug’s efficacy in slowing ALS progression. These measures included self-reported physical activity and independent eating and drinking ability, wearable device data on activity, and physician-measured changes in mobility, muscle strength, and lung function.
The results showed that patients who received ropinirole throughout both phases of the trial were more physically active compared to the placebo group. They also exhibited slower rates of decline in mobility, muscle strength, and lung function, and had a higher survival rate.
While the trial indicates the potential therapeutic effect of ropinirole in ALS patients, further studies, including a phase 3 trial, are required to confirm its effectiveness. Nonetheless, these findings offer hope for ALS patients, as there is currently no cure for the disease, and treatment focuses on symptom reduction and supportive care.
The effectiveness of ropinirole compared to the placebo became increasingly evident as the trial advanced. However, patients in the placebo group who switched to ropinirole midway through the trial did not experience similar improvements, suggesting that early initiation and longer duration of ropinirole treatment may be crucial for its efficacy.
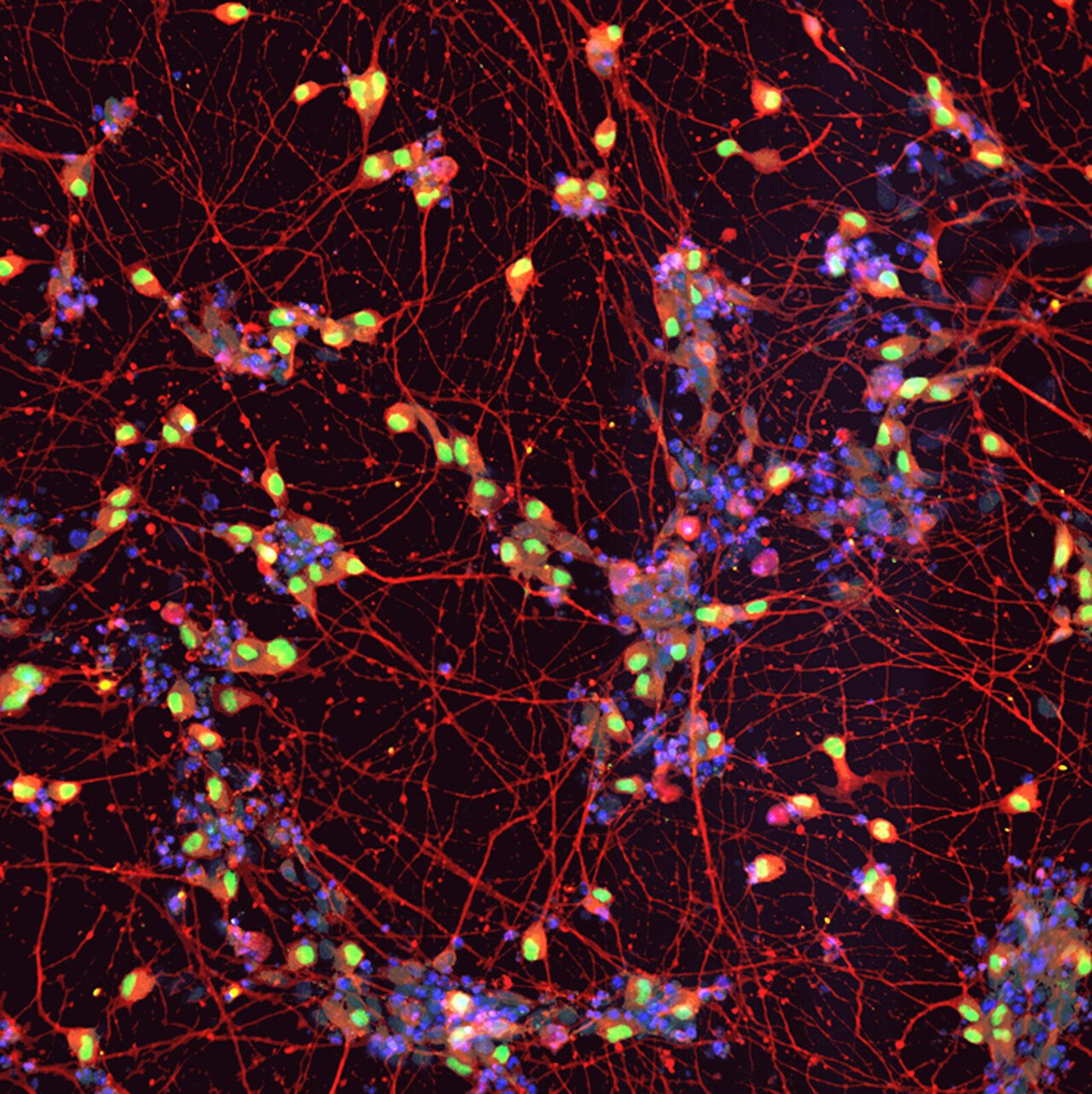
In order to understand the underlying mechanisms of ropinirole’s effects and identify molecular markers of the disease, the researchers utilized induced pluripotent stem cells derived from the patients’ blood and cultivated these cells into motor neurons in the laboratory. They observed notable differences in structure, gene expression, and metabolite concentrations between motor neurons derived from ALS patients and those from healthy individuals. However, treatment with ropinirole reduced these differences.
Specifically, motor neurons derived from ALS patients exhibited shorter neurites compared to healthy motor neurons. Yet, when treated with ropinirole, these axons grew to a more normal length. The research team also identified 29 genes associated with cholesterol synthesis that were upregulated in ALS patient-derived motor neurons. However, ropinirole treatment progressively suppressed the expression of these genes. Additionally, lipid peroxide emerged as a promising surrogate marker for estimating the effect of ropinirole both in laboratory experiments and in clinical settings.
The researchers discovered a significant correlation between a patient’s clinical response to ropinirole and the response of their motor neurons in vitro. Patients whose motor neurons demonstrated a robust response to ropinirole in the laboratory experienced slower disease progression with ropinirole treatment. On the other hand, patients with suboptimal motor neuron response displayed faster disease progression despite taking ropinirole.
This suggests that the method employed in this study, involving the cultivation and testing of motor neurons derived from induced pluripotent stem cells, could potentially be utilized in a clinical setting to predict the efficacy of ropinirole for individual patients. Although the reasons behind variable responsiveness to ropinirole among patients remain unclear, the researchers aim to identify genetic differences that may contribute to this phenomenon in future investigations.
Source: Cell Press