Chemists have long been challenged by the task of controlling chemical reactions to create new products. The ability to manipulate reactions has far-reaching implications for various industries, such as reducing waste in construction material manufacturing or enhancing catalyst production for faster reactions.
In the realm of polariton chemistry, which combines the tools of chemistry and quantum optics, researchers have been conducting experiments in optical cavities over the past decade. These experiments aim to manipulate the chemical reactivity of molecules at room temperature using electromagnetic fields. While some success has been achieved in altering the products of chemical reactions in organic compounds, no research team has been able to establish a consistent physical mechanism to describe and reproduce the phenomenon, especially in the last two years.
However, a breakthrough has been made by a collaborative team of researchers from Universidad de Santiago in Chile, affiliated with the Millennium Institute for Research in Optics (MIRO), and the chemistry division of the US Naval Research Laboratory. Led by principal investigator Felipe Herrera and researcher Blake Simpkins, respectively, the team has published their findings in the prestigious journal Science on June 16, 2023.
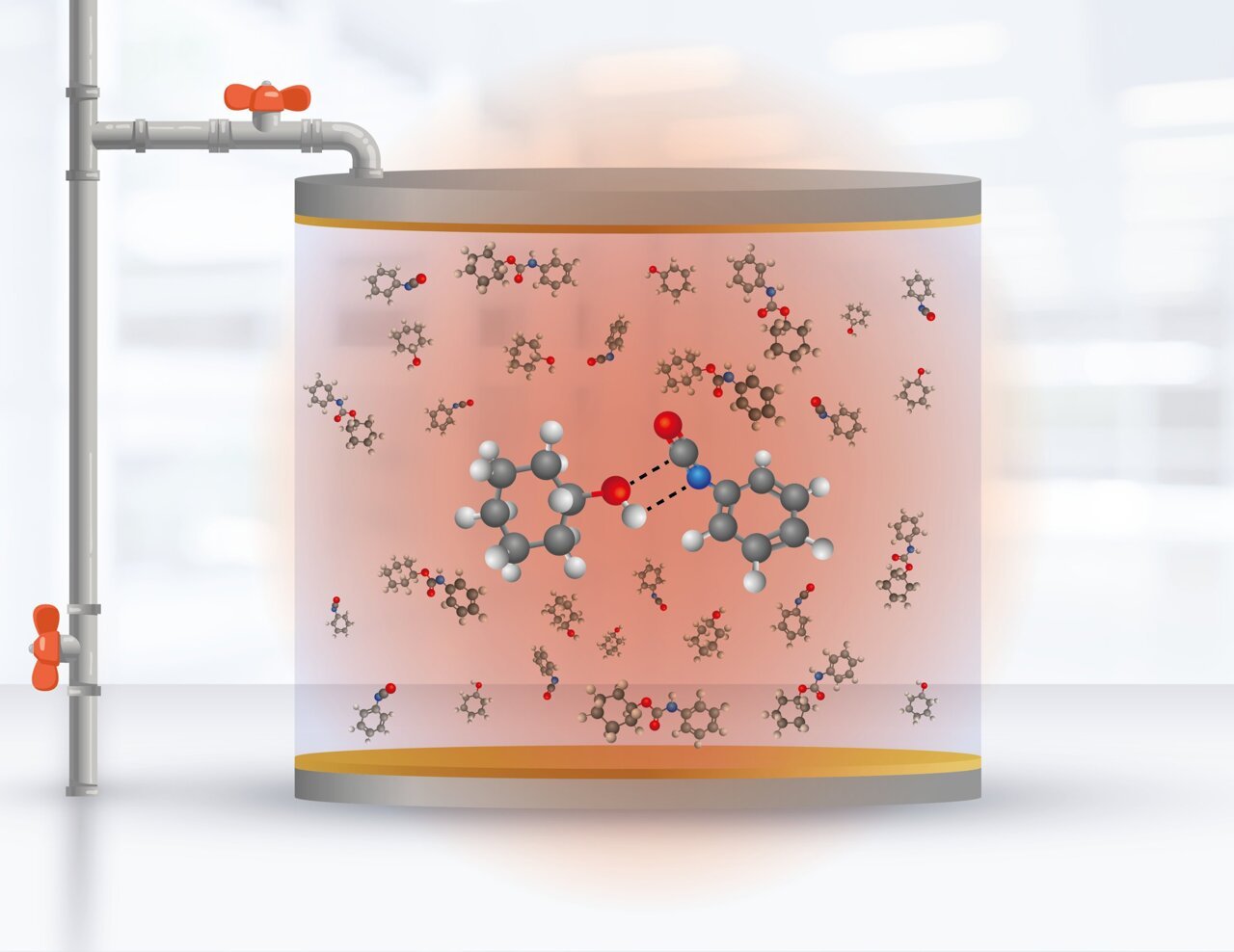
The researchers have successfully demonstrated, both theoretically and experimentally, the manipulation of the formation rate of urethane molecules in a solution contained within an infrared cavity. This groundbreaking discovery provides compelling evidence that it is indeed possible to selectively modify the reactivity of specific bonds in a chemical reaction at room temperature and in a liquid solvent. The key factor behind this achievement is the influence of the electromagnetic field vacuum within a narrow range of infrared frequencies.
Felipe Herrera, one of the lead researchers, emphasizes the significance of this theoretical breakthrough. He states that their findings improve the fundamental understanding of this phenomenon compared to other models that only explain partial aspects of experimental observations or dismiss the evidence entirely.
New scientific scope for molecule manipulation
Controlling chemical reactions is a challenging task due to the intricate nature of the underlying processes. When a chemical reaction occurs, the bonds holding atoms together within a molecule are broken and rearranged, resulting in the formation of new substances, or products. This rearrangement requires energy, and the principles of thermodynamics, established in the 19th century, help us understand how energy transfers take place during these reactions.
Furthermore, there are reactivity principles based on molecular structures, such as those proposed by Eyring, Evans, and Polanyi in 1935. These principles have been widely applied in all branches of chemistry and assume that each reaction between two molecules is independent of other chemical reactions occurring in a solution. For nearly eight decades, these principles have been valid in the majority of studied situations. However, the presence of the electromagnetic vacuum introduces correlations between different chemical reactions within the confined space of the cavity, thereby challenging the traditional assumptions of chemical reactivity, as explained by Felipe Herrera.
The significance of the experimental findings lies in confirming the modification of reaction rates through the interaction with the electromagnetic vacuum within the cavity. The study focused on a well-studied chemical reaction and demonstrated more pronounced changes compared to other reaction types. In the theoretical aspect, the contribution lies in the ability to control the resulting products by modifying the dynamics of the key chemical bonds involved in the reaction through the influence of the infrared vacuum. Johan Triana, a postdoctoral fellow at MIRO and the University of Santiago, played a role in developing the mathematical model and performing numerical calculations to describe the molecular system.
Reproducing and interpreting measurements
The research project was initiated in 2020 by Dr. Wonmi Ahn, who was a postdoctoral fellow at the US Naval Research Laboratory at that time. In 2021, Blake Simpkins took charge of preparing new samples to ensure the reproducibility of measurements and improved the liquid cells used for the chemical reactions.
During the same year, researcher Felipe Herrera began collaborating with Simpkins, holding regular meetings to explore possible theoretical explanations to support the experimental results. They decided to construct a theory from the ground up, incorporating all the physical aspects of quantum optics while ensuring it could be reduced to the standard reactivity theory of theoretical chemistry under specific conditions. Felipe Herrera, a professor at MIRO and Universidad de Santiago de Chile, elaborates on this approach.
The culmination of their collaborative efforts resulted in the publication titled “Modification of ground-state chemical reactivity via light-matter coherence in infrared cavities.” The paper was co-led by Simpkins from the US Naval Research Laboratory and Herrera from MIRO, Universidad de Santiago de Chile. The research also involved the participation of researcher Wonmi Ahn from Bilkent University in Turkey, as well as researcher Johan Triana and Ph.D. student Felipe Recabal, both associated with the Molecular Quantum Technology group of MIRO at Universidad de Santiago de Chile.
This initial work opens up new avenues and scientific challenges, according to Dr. Herrera. He emphasizes the need to develop a comprehensive yet accessible theoretical and mathematical framework that researchers worldwide can employ to interpret their own experiments and potentially devise novel measurement techniques that have yet to be envisioned.
In this regard, Herrera contemplates his aspirations as a scientist bridging the fields of physics and chemistry, expressing his desire to establish a consistent theory that unifies two of the most successful disciplines in modern science: chemical kinetics and quantum physics.
Source: Millenium Institute for Research in Optics