Renewable energy solutions are crucial in our quest to meet increasing energy demands while addressing environmental concerns and achieving net-zero emissions by 2050. One promising technology in this field is microbial electrochemical technology, particularly microbial fuel cells known as SMFCs. These systems are not only cost-effective but also environmentally friendly, making them highly attractive for green energy systems. By harnessing the power of endogenous microorganisms found in soil, SMFCs convert organic matter into electricity, providing a sustainable energy source while simultaneously offering a self-powered bioremediation strategy for contaminated soils.
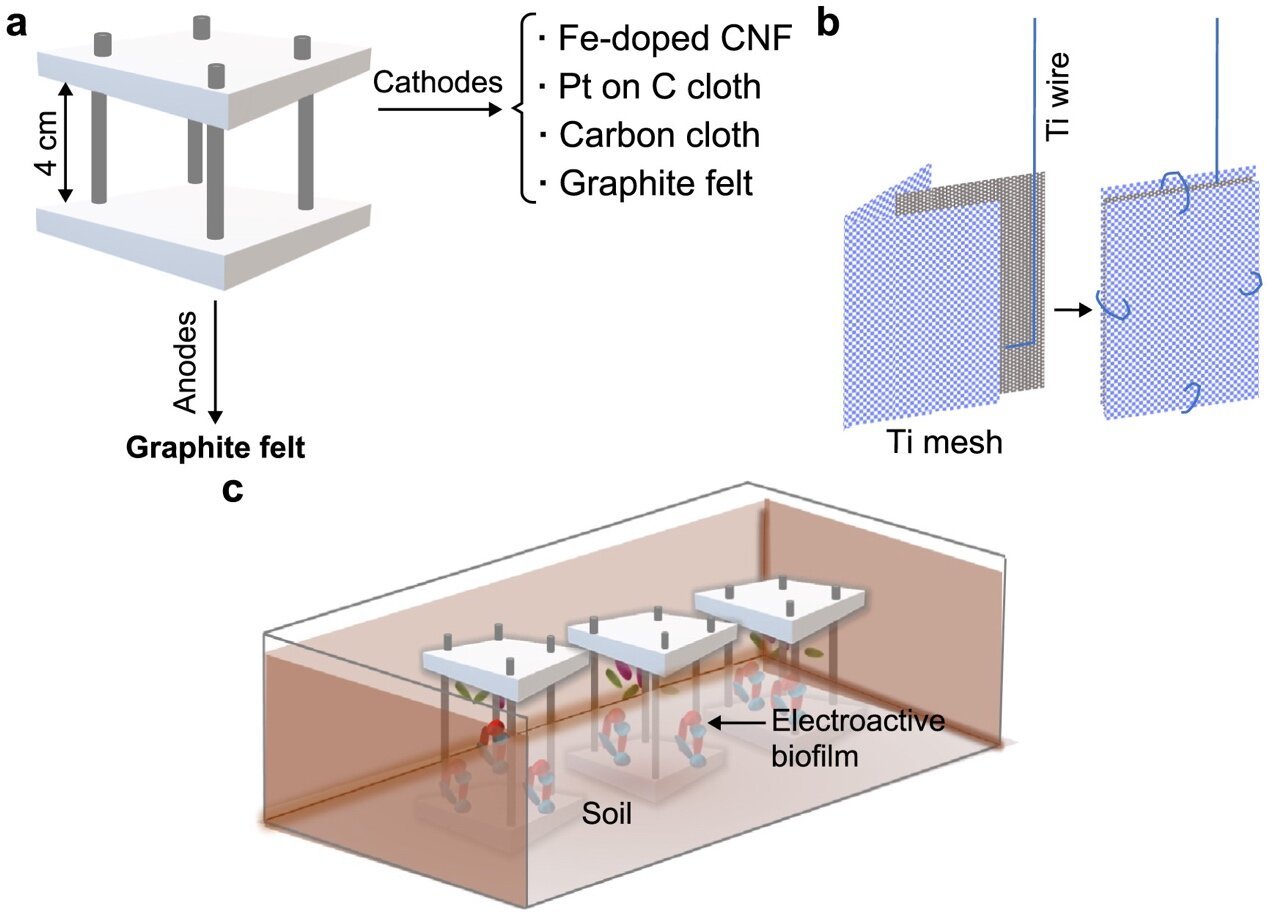
The performance of microbial fuel cells heavily relies on the cathode materials utilized. Recently, researchers conducted a study comparing the performance of membrane-free air-cathode SMFCs using four different cathodes: carbon cloth, Pt-doped carbon cloth, graphite felt, and an innovative Fe-doped carbon nanofibers electrode. To evaluate the impact of these electrode materials on biofilm formation and electrochemical performance, the researchers conducted extensive electrochemical tests over prolonged periods and performed microbial taxonomic analyses.
The findings of this study, published in the Environmental Science and Ecotechnology journal by researchers from the University of Bath, revealed that Fe-doped carbon nanofibers and Pt-doped carbon cloth cathodes exhibited stable performances, achieving peak power densities of 25.5 and 30.4 mW m�?�2, respectively. Graphite felt cathodes demonstrated the best electrochemical performance among all the tested materials, with a peak power density of 87.3 mW m�?�2. However, these cathodes also displayed the highest level of instability.
Microbial community analysis showed variations between anodic and cathodic communities. Anodes were primarily enriched with Geobacter and Pseudomonas species, while cathodic communities were dominated by hydrogen-producing and hydrogenotrophic bacteria. This suggests that hydrogen cycling could potentially serve as an electron transfer mechanism. Furthermore, the presence of nitrate-reducing bacteria, coupled with cyclic voltammogram results, indicated that microbial nitrate reduction occurred specifically on graphite felt cathodes.
The use of innovative Fe-doped carbon nanofiber cathodes demonstrated electrochemical performance comparable to Pt-doped carbon cloth, providing a low-cost alternative. However, graphite felt outperformed all other tested electrodes in terms of electrochemical performance, despite showing lower reproducibility and higher mass transport losses. Microbial taxonomic profiling of the cathode biofilms revealed the presence of taxa associated with oxygen reduction and the utilization of alternative electron acceptors, such as nitrate.
The outcomes of this study hold significant implications for future research on low-cost, high-performing SMFCs for practical applications in energy harvesting and bioremediation. By gaining a deeper understanding of how electrode materials influence microbial communities and electrochemical performance, researchers can expedite the development and implementation of SMFCs in real-world scenarios.