Malaria continues to be a significant public health issue, particularly in sub-Saharan Africa, with nearly 250 million cases and 621,000 fatalities reported each year. This parasitic disease, transmitted by mosquitoes and caused by the Plasmodium microbe, requires specific adaptations as it travels from mosquito to human, invading various organs and cells. Unlike sensory organs found in other organisms, microbes utilize protein-based sensors to detect specific molecules in their environments. Plasmodium, however, stands out with its unique set of sensors.
Researchers at the University of Geneva (UNIGE) have made an exciting discovery regarding Plasmodium’s sensing abilities. They have identified a novel sensor that enables the parasite to precisely determine its location and respond accordingly. Published in the journal Science Advances, this breakthrough opens up the potential for disrupting the signals detected by this sensor, thereby disorienting the parasite and impeding its replication and transmission.
When an individual is bitten by a mosquito carrying Plasmodium, the parasite enters the bloodstream and makes its way to the liver, where it thrives silently for approximately 10 days without causing any symptoms. After this initial phase, Plasmodium re-enters the bloodstream, infiltrating and multiplying within red blood cells. Following a synchronized 48-hour cycle, the newly-formed parasites burst out of their host cells, destroying them in the process and infecting new red blood cells. This destruction of red blood cells gives rise to the characteristic fever waves associated with malaria. In severe cases, the blockage of blood vessels by infected red blood cells leads to complications.
When a mosquito subsequently bites an individual with Plasmodium-infected blood, the parasite adapts its development program to colonize the mosquito’s intestine. After further multiplication, Plasmodium migrates back to the salivary glands of the mosquito, primed to infect a new human host.
Unknown communication channels
The journey of Plasmodium from the warm confines of a red blood cell to the depths of a mosquito’s intestine involves a remarkable ability to perceive changes in its environment and adapt its development program accordingly. Understanding this intricate biological mechanism is crucial for devising strategies to combat this parasite, as explained by Mathieu Brochet, Associate Professor in the Department of Microbiology and Molecular Medicine at UNIGE Faculty of Medicine, who led the research project. The question arises: How does Plasmodium discern the signals it needs to react appropriately at each stage of its life cycle?
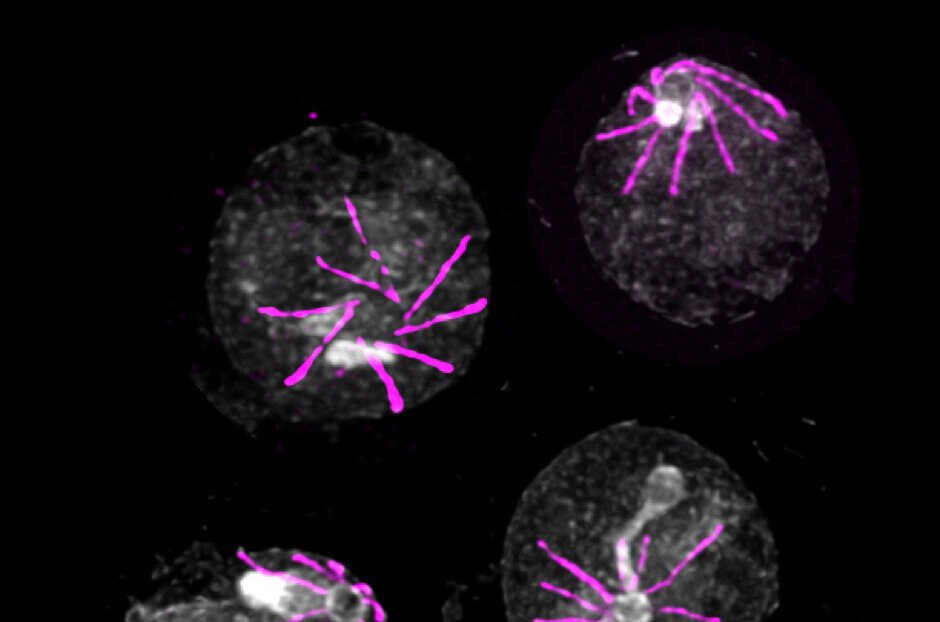
Researchers have uncovered that Plasmodium can detect specific small molecules that are absent in the bloodstream but present in the mosquito. “Starting from this key element, we have identified a sensor that allows the parasite to perceive the presence of these molecules when it enters a mosquito,” elaborate Ronja Kühnel and Emma Ganga, Ph.D. students in Mathieu Brochet’s laboratory and the primary authors of the study. This sensor comprises five proteins, and in its absence, the parasite remains oblivious to its transition from the bloodstream to the mosquito, hindering its further development.
Remarkably, this sensor is also active during other stages of the parasite’s lifecycle, particularly when it needs to exit the red blood cell. “We observe the exact same mechanism in this scenario: without the sensor, Plasmodium becomes trapped inside red blood cells, unable to continue its infectious cycle,” explain the scientists. However, the specific human molecules detected by the parasite have yet to be identified. Unraveling these molecules could enhance our understanding of how Plasmodium induces fever waves during infection.
The protein complex discovered in this study is absent in humans but is found in the broader family of apicomplexan parasites, including Plasmodium and Toxoplasma (the causative agent of toxoplasmosis). With the identification of this sensor, scientists can now explore methods to disrupt the signals perceived by the parasite at different stages of its development. By scrambling these signals, the aim is to disorient the parasite, impeding its multiplication and transmission.
Overall, these findings provide valuable insights into the intricate sensory mechanisms employed by Plasmodium and offer new avenues for developing interventions to combat malaria and related diseases caused by apicomplexan parasites.
Source: University of Geneva